Chemical resistance
Introduction
The term “chemical resistance” is normally used, although an absolute resistance is never given.
The chemical resistance describes how the resistance properties of polymers mutual interact with the chemical agents (e.g. air, gas, water, grease).
This means that the substances influence the polymers, but at the same time also the polymers infl uence the chemical agents. For example, some polymers affect on the grease consistency, and thus the grease infl uence negatively the polymers.
Effect on the plastic
Chemical interaction agents react with the primary valence bonds and modify the polymers´ properties irreversibly. In this case the chain breaks, thus it shortens and induces an embrittlement of the polymers. The polymers response with softening, swelling till its dissolution in the agent. Even a small chemical attack could lead to strong properties changes.
Physical interaction agents do not react with the polymers, but they reduce their physical bonds forces. The agent penetrates in the empty areas of the chains, spreads them and increases the space between the chains, thus it causes a reduction of the secondary valence bonds forces. This reaction takes place till the polymers reach a state of equilibrium. The polymers respond with softening and swelling. If the agent is extracted from the polymers, they will nearly restore the initial properties.
Physical interaction agents influence are used, e.g. by the polyamides conditioning. The polyamides, with an absorption of water (agent), pass from a hard and brittle condition to an elastic and tough condition.
Influencing parameters
The influence intensity of the agents depends on the following parameters:
Temperature
A temperature increment leads to a speed increment of the Brownian motion. The processes go every 10°C temperature increment, from 3 up to 4 times faster.
If the temperature rises above the glass transition temperature, a sharp reduction of the resistance occurs.
Residence time
With the increment of the residence time decreases the resistance.
Concentration
In aqueous solutions, the resistance decreases with a concentration increment.
Degree of crystallinity
Through the crystallization, a big part of the crystalline structure increases its resistance, thanks to a more densely packed crystalline structure. Consequently the penetration of agents will be more difficult.
Filler materials
Filler materials as e.g. fibres reduce the resistance. Fibres act like capillary, and enable a faster penetration of the agents into polymers.
On the other hand, fibres lead to a structure deformation reduction and, consequently, to an increment of the stress cracking resistance.
Polymer internal stress level
The internal structure, through internal tensions, will deform and by doing so it will create free space between the chains, and therefore it will easily permit the penetration of agents. Thus the resistance will be reduced. These free spaces created by internal tension, are many times bigger in amorphous structures than in crystalline structures. This causes the lower resistance of the amorphous materials (see “Resistance to stress cracking”).
Resistance to stress cracking
When amorphous polymers are exposed to tensions, these can also be internal tensions caused by processing, the lattice structure will change.
This generates free spaces, thus it produces stress cracks and therefore permit an easy and deep penetration of agents in the amorphous polymers.
At this point deep cracks are developed, and this leads the workpiece to the breakdown. Only with mechanical tensions or only with agents, such a damage does not take place. In amorphous polymers, as for example ZX-410 and ZX-410V7T, the resistance to stress cracking must be checked, before using them with chemicals.
In these cases, please contact us.
Chemical resistance of the ZEDEX® engineering & high-performance polymers
To determine whether a ZEDEX® engineering & high-performance polymer is resistant to a specific chemical substance or not, the following 5 possibilities are at your disposal:
1. Relative values
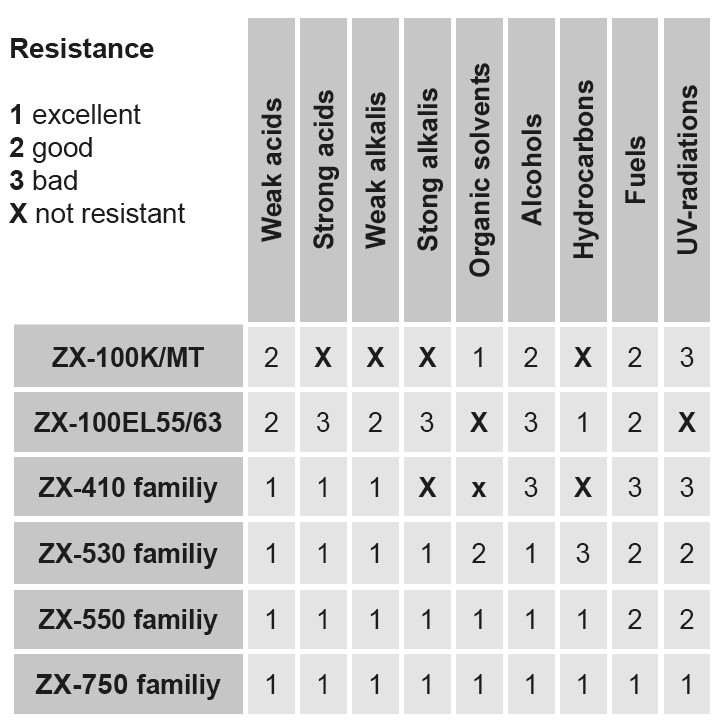
2. Chemicals list - when chemicals names are known
In the alphabetical order arranged list of chemicals, the ZEDEX® materials´ resistance to the listed chemicals, depends on their concentration and on the application temperature.
3. Synonym list - if chemicals are not available in the chemicals list
The chemicals are often be known with various names. In the synonym list, these chemical synonyms are listed keeping the order of the chemicals present in the chemicals list.
4. Chemical groups list - if chemicals are neither in the chemical nor in the and synonym lists
The chemicals can be classifi ed into chemical groups, using their chemical formula. These chemical groups, as well as some typical examples, are listed in this chart
| Chemical group | Typical representatives |
|---|---|
| Aldehydes / ketones | acetaldehyde |
| acetone | |
| Methyl ethyl ketone | |
| Alcohols / glycols | Diethylene glycol |
| Ethanol | |
| Glycerin | |
| Isopropanol | |
| Methanol | |
| Trichloroethanol | |
| Aliphatic hydrocarbons | acetylene |
| methane | |
| Octane | |
| Amides | Acetamide |
| Dimethylacetamide | |
| Dimethylformamide | |
| Formamide | |
| Amines | aniline |
| Dimethylamine | |
| Ethylenediamine | |
| Triethylamine | |
| Aromatic hydrocarbons | benzene |
| toluene | |
| Halogenated hydrocarbons | Carbon tetrachloride |
| Fluorine-chlorine-hydrocarbon (FCK) | |
| Tetrachlorethylene | |
| Trichloroethane (1,1,1-) | |
| Trichlorethylene | |
| Esters | Amyl acetate |
| Ethyl acetate | |
| Ether | Ether |
| Isopropyl ether | |
| Halogens | Chlorine (liquid) |
| Chlorine gas (dry) | |
| Inorganic acids | hydrochloric acid |
| phosphoric acid | |
| sulfuric acid | |
| Inorganic alkalis | Ammonium hydroxide |
| Caustic soda | |
| Nitriles | Acetonitrile |
| Acrylonitrile | |
| Organic acids | acetic acid |
| Formic acid | |
| Oleic acid | |
| Phenols | phenol |
| Inorganic salts | Potassium carbonate |
| Potassium chlorate | |
| Potassium chloride | |
| Potassium sulfate |
5. pH-limit value list - resistance to inorganic acids, alkalis and salts
With the help of the pH limit list the chemical resistance of plastics to inorganic acids, alkalis and salts can be determined. The pH values of some substances are listed in the table on the left as typical examples. In addition, the limit values for ZEDEX® materials can be found in the second table below. If the pH of the chemical used is between the two limit values, the material is very likely to be resistant to the chemical.
| substance | pH value | Type |
|---|---|---|
| Battery acid | < 0 | acidic |
| Stomach acid (empty stomach) | 1,0 - 1,5 | |
| Lemon juice | 2,4 | |
| cola | 2,0 - 3,0 | |
| vinegar | 2,5 | |
| Black morello fruit juice | 2,7 | |
| Orange and apple juice | 3,5 | |
| Wine | 4 | |
| Sour milk | 4,5 | |
| beer | 4,5 - 5,0 | |
| acid rain | < 5,0 | |
| coffee | 5 | |
| tea | 5,5 | |
| Rain (natural precipitation) | 5,6 | |
| Mineral water | 6 | |
| milk | 6,5 | |
| Water (depending on hardness) | 6,0 - 8,5 | acidic to alkaline |
| Human saliva | 6,5 - 7,4 | |
| blood | 7,4 | alkaline |
| Sea water | 7,5 - 8,4 | |
| Pancreatic juice (intestinal juice) | 8,3 | |
| Soap | 9,0 - 10,0 | |
| Household ammonia | 11,5 | |
| Bleach | 12,5 | |
| concrete | 12,6 | |
| Caustic soda | 13,5 - 14,0 |
| material | pH value lower limit | pH value upper limit |
|---|---|---|
| ZX-100K | 1 | 9 |
| ZX-100EL55 | 1 | 9 |
| ZX-100EL63 | 1 | 9 |
| ZX-100MT | 1 | 9 |
| ZX-324 | 1 | 14 |
| ZX-324V1T | 1 | 10 |
| ZX-324V2T | 1 | 14 |
| ZX-324V3T | 1 | 14 |
| ZX-324V11T | 1 | 10 |
| ZX-324VMT | 1 | 14 |
| ZX-410 | 1 | 9 |
| ZX-410V7T | 1 | 9 |
| ZX-410VMT | 1 | 9 |
| ZX-530 | 1 | 14 |
| ZX-530CD3 | 1 | 14 |
| ZX-530EL3 | 1 | 14 |
| ZX-530EL3AG2 | 1 | 14 |
| ZX-530KF15 | 1 | 14 |
| ZX-550 | 1 | 14 |
| ZX-550PV | 1 | 14 |
| ZX-750V5T | 1 | 9 |
| ZX-750V5KF | 1 | 9 |
Sterilisation
The word sterilization describes the procedure to eliminate the microorganisms from materials and items.
After this procedure, the materials and items are called with the term “sterile”. Through the sterilization of the items, would be theoretically destroyed all the microorganisms that belong to or hang on the items, therefore killing the spore forms, viruses, prions (proteinaceous infectious particle), plasmids and other DNA-fragments.
In practice, a full sterilization is not achievable with a reliability of 100 %. Therefore we can talk about a reduction of the microorganisms number of a specific factor (in powers of 10), depending on the application required, or about a possibility of full sterilization.
For example, it is possible to require, that the residual content of the microorganisms of a sterilized item is maximum 10−6 c.f.u. (colony forming units). That means, that the possibility of fi nding a microorganism
in the item, is maximum one in a million. Looking at the technical defi nition of disinfection, it can be noticed that generally the sterilization has got a
bigger probability (in power of 10) of a full sterilization.
The sterilization is obtainable through physical (thermal, radiation) or chemical procedures.
Chemical sterilisation
The term “chemical sterilization” refers to a sterilization made through specifi cs chemical substances, as formaldehyde or peracetic acid.
The chemical sterilization is normally used for thermolabile materials.
With thermostable materials, it is always preferable a steam sterilization to a chemical sterilization.
Liquid chemical sterilization
The destruction of microorganisms is done by the application of chemicals, some of them in a liquid form, on the items to be sterilized.
For example, the sterilization in the beverage technology, is made with hydrogen peroxide, dissolved ozone or peracetic acid. A critical parameter in all the liquid chemical sterilization processes, is the temperature of the sterilizing solution.
To remove the chemicals from the sterilized object, this one is typically washed with sterile water.
Dry sterilization process
The microorganisms killing is done with gas on the dry item to be sterilized. Gas sterilization is performed for example with formaldehyde, ethylene oxide, ozone or hydrogen peroxide.
This procedure occurs frequently in the cold antiseptic fi lling of foods, especially in beverages. Before filling the to be sterilized objects, mostly plastic bottles made of PET or HDPE, these ones are sterilized first with chemical agents, mostly with peracetic acid products, then washed (liquid chemical sterilization process) and as final treatment, for a further killing of microorganisms, additional gases are used, preferably hydrogen peroxide in gaseous form. [*]
[*] https://de-academic.com/dic.nsf/dewiki/1330984%E2%80%98Chemische_Sterilisation
Moist heat sterilization
The moist heat sterilization (heating in an autoclave) is the standard procedure. The air inside the autoclave is completely replaced by water steam. The real time and the temperature necessary for a sterilization process depends on the autoclave type and on the pathogens agents´ resistance.
The item is heated 20 minutes at 121 °C with a water steam’s pressure of 2 bar, or 5 minutes at 134 °C with a pressure of 3 bar. To eliminate the prions, a temperature of 134 °C, with 3bar pressure and a time of 18 minutes are required. [*]
* https://de-academic.com/dic.nsf/dewiki/1330984%E2%80%98Chemische_Sterilisation
Dry heat sterilization
- The annealing of metal objects through a temperature of about 500 °C, is commonly used in microbiological laboratories.
- The flame treatment: the item is subjected for few seconds to a flame treatment.
- The dry heat sterilization of glass, metals, porcelain are made with one of the following procedures:
180 °C for at least 30 min.
170 °C for at least 60 min.
160 °C for at least 120 min.
Radiation sterilization
In the industrial sterilization process (as the medical disposables) gamma irradiations or electron beams
are used in large-scale. [*]
* https://en.wikipedia.org/wiki/Sterilization_(microbiology)
Reaction of the polymers
Depending on the polymer type, the reaction mechanisms to the action of high-energy radiation, consist of chain splitting, chain branching or crosslinking.
As a result of these reactions, the following changes can also occur weeks after the radiation procedure:
- discoloration
- gas separation
- odour formation
- crosslinking
- embrittlement
- strengthening
- softening
- chemical decomposition
- molar mass variation
- improvement or deterioration of the mechanical
and chemical properties - change in the melting and glass transition
temperature - toxicological changes.
High energy radiation
The radiation absorbed dose is defined as the amount of energy absorbed per unit mass.
SI unit is [J / kg] or [Gy]; the old unit was [Rad], abbreviated “rd”.
The conversion is the following: 1 J / kg = 1 Gy = 100 rad. The absorbed dose rate indicates how fast the energy is absorbed [Gy / s].
Between a sterilization done either by electron beam, or X-ray or gamma-ray, there are no direct differences, concerning their infl uence on the plastic properties variations, of course when these procedures have got the same absorbed dose and dose absorbed rate. Anyway, there is an indirect difference between a sterilization made with gamma irradiation and one made with an electron beam. In fact in both procedures an oxidative degradation takes place.
However with the same absorbed dose and dose absorbed rate, this degradation is with an electron beam many times higher than with a gamma irradiation.
Relative resistance against high energy radiation
Following figure shows the pure radiation resistance of the polimers. As property limit value, an elongation at break decrement of –25% from the initial value is used.
Looking at this graphic is observable, that thermosetting plastic are more resistant than the thermoplastic, and aromatic polymers are more resistant than the aliphatic ones. Polymers with low density have got a higher resistance.

Rating of ZEDEX® polymers resistance against radiation
ZX-100K is relatively resistant to high-energy radiation. A first strong degradation occurs with an absorbed dose of 1000 kGy (10 kGy / h; 1 meV gamma ray, source: Cobalt-60).
ZX-410 has got a high resistance to gamma-rays and beta-rays radiations. After a radiation exposure of 15000 kGy (2 MeV electron beam; 5 kGy / s), ZX-410 has still got 90 % of its tensile strength.
ZX-324V1T is to alpha, beta and gamma rays very resistant. A gamma-ray absorbed dose of 10 000 kGy causes nearly no damage.

What is your challenge today?
Ask the right questions to let us develop the ideas to win it!
